Electrolyser - Hyjack : Hydrogen Online
Log in to start sizing and costing the Electrolyser.
About Tech
Hydrogen Production by Electrolysis of Water
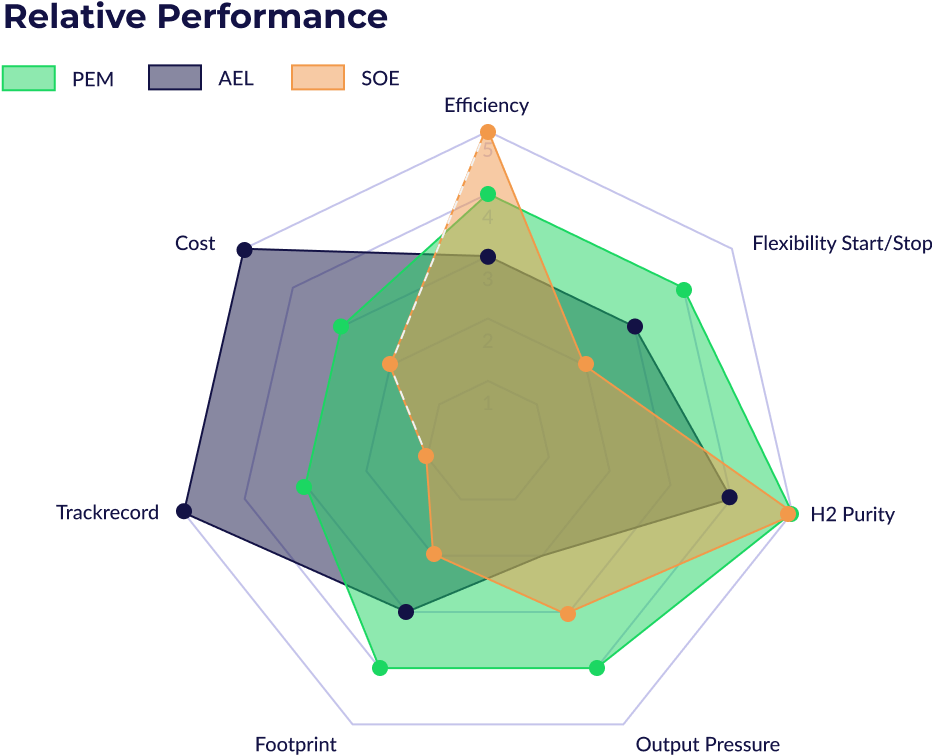
Polymer Electrolyte Membrane (PEM) and Alkaline (AEL) electrolysers are both mature and reliable technologies. Their respective characteristics and suitability according to the utilisation are compared on this radar graph. Alkaline technology is better in terms of cost and track record while footprint and reactivity talk for PEM. Electrolyser reactivity is of interest when associated with solar or wind electricity sources (the capacity factor slider in the calculator allows to consider the typical load factor of renewables).
- AEL is the most mature electrolyser technology. It operates at temperatures between 60°C and 90°C and
reaches up to 35 bar of pressure. Electrodes are submerged in a liquid electrolyte/ionic conductor,
typically a 25-30% aqueous KOH-solution, which is a conductive corrosive solution.
The solution is circulated either by a pump or by the natural circulation occurring due to
the temperature gradients and/or movement of gas bubbles. The electrolyte is stored in two different
chambers for each product gas (O2 and H2) separated by a solid barrier called a diaphragm.
- PEM is a built up around a proton exchange membrane in the middle of the cell and consists of an anode
for oxygen production and a cathode for hydrogen production. The design of the PEM is compact,
it operates at temperatures between 50°C and 85°C and due to the solid assembly, its operation supports
high-pressure conditions and are typically operating around 70 bars. Typically, the electrodes are in
direct contact with the proton exchange membrane. At the anode, water is oxidized to produce oxygen,
electrons, and protons that circulate across the membrane to the cathode where they are reduced,
closing the circuit and producing hydrogen that bubbles towards the cathodic gas outlet.
In most cases a highly conductive Nafion proton membrane will separate the two chambers and
the electrodes are typically applied directly to the membrane, which makes up the membrane electrode assembly.
The corrosive acidic solution in the PEM requires noble metal catalysts like iridium for the anode and
platinum for the cathode. The membrane features a very low cross-permeation of the gases, producing
hydrogen with a high purity after drying.
- SOE is a technology that enables water, or more specific steam electrolysis, to operate at high
temperatures ranging from 600°C to 1000°C with a pressure between 15 and 25 bars.
It is a highly efficient technology in which an electric current is passed through two electrodes,
which can be made up of relatively cheap nickel, separated by an electrolyte. Electrolysis of steam
convert electric energy and heat into hydrogen and oxygen. In the overall process for steam electrolysis
gaseous water is fed to the negative electrode where it is split into hydrogen and oxide ions.
The oxide ions are conducted through the solid oxide electrolyte from the negative electrode to
the positive by the applied electric field. At the positive oxygen electrode, the oxide ions
recombine to produce gaseous oxygen.
At The Heart Of The Challenge : The Electrolyser Efficiency
The global efficiency of the electrolyser is the result of several sub level efficiencies. More precisely, it is a function of (a) the DC efficiency representative of the stack efficiency, the very core of the process (which can be divided in faraday and voltage efficiencies) and (b) the efficiency of all the auxiliaries necessary to run an electrolyser system, it can include the cooling, H2 purification, water treatment and AC conversion among other.

At HyJack we use a global system efficiency for our calculator. The definition of the system boundaries vary from a manufacturer to another and deserve to be specified when comparing quotes. For now, in the tool, the efficiency follows a curve specific to each given technology. Note that the efficiency varies because of different specifications used by each manufacturer. It is also sensitive to the outlet pressure and the specific needs of the underling project (such as cooling and gas treatment). In particular, small unit efficiency is typically lower due to a higher proportion of electricity needs related to auxiliaries.
| Stack | ALE | PEM | Units |
|---|---|---|---|
| 61-84 | 64-84 | % HHV | |
| 4.2-5.9 | 4.2-5.5 | kWh/Nm3 | |
| System | ALE | PEM | Units |
| 55 - 79 | 55 - 84 | %HHV | |
| 4.5 - 6.6 | 4.2 - 6.6 | kWh/Nm3 |
HyJack electrolyser efficiency equations
AEL
Specific consumption (kWh/Nm3) = 6.04*power-0.02
PEM
Specific consumption (kWh/Nm3) = 6.35*power-0.028
SOE
Specific consumption (kWh/Nm3) = 3.26*power-0.009
For the SOE electrolyser, the model considers that 20 % of the total energy needed by the electrolysis system is supplied in the form of heat. Therefore, the total HHV power efficiency for this technology can exceed 100 %.
About costing
The significant impact of the scale effect
The electrolyser cost is subject to a scale effect, the cost per kw installed
decreases significantly until 10 MW. The cost here is representative of an
electrolyser
providing hydrogen
at 30 bar. While atmospheric pressure electrolyser exists (alkaline mainly) and can
be
an
economic alternative,
it fails to meet the current industry practices and represents additional
compression
need for
storage.
The costing is expressed at two different levels:
- The equipment cost, includes the stack and basic balance of
plant.
The costing (€/kW hhv or $/kW hhv) equation is function of the nominal power of the
electrolyser.
- The total cost represents the sum of the equipment cost and the
auxiliary
costs.
The latter can be significative, it includes an estimation of engineering, civil
works,
transportation, instrumentation and piping costs. Total cost is expressed as a
range,
with a
low and a high estimations both calculated as a percentage (%) of the equipment
cost.
The low estimate of the total electrolyser cost is set to a sum of the equipment
cost
plus
an additional 70%. The high estimate of the total cost includes the
equipment
cost plus an additional 130%.
Note that the total cost doesn't include contingencies and owner's costs.
HyJack electrolyser cost equation
Last update 08/01/2022
AEL
Equipment cost(€/kW) = 4841*power-0.198
PEM
Equipment cost(€/kW) = 6046*power-0.2
SOE
Equipment cost(€/kW) = 142790*power-0,548
SOE is a technology still under development, the associated cost curve is a medium-long term projection.
Keep in mind the stack replacement costs
The stacks will probably need to be renewed after their expected lifetime (approx. 80 000 hours for Alkaline, 60 000 hours for PEM and 20 000 hours for SOE). The cost breakdown shows an estimation of the investments necessary to changing the stacks. Note that the share of the stack is slightly more important for a PEM electrolyser.
PEM SYSTEM COST COSTBREAKDOWN (%)
AEL SYSTEM COST COSTBREAKDOWN (%)
''
The cost curves here represent a fair estimate of the average price. Given
the
diversity
of
suppliers and the product standardisation being still in its early stages,
there
remains
a
significant dispersion of actual prices between suppliers and projects.
Optimal calibration of the asset with the exact outputs goes beyond the
scope of
this
platform. Precise engineering and costing should be subject to case-by-case
discussion with the suppliers.

Sources
REPORTS:
- IRENA (2019), Innovation landscape brief: Renewable Power-to-Hydrogen, International Renewable Energy Agency.
- IAE (2019), The Future of Hydrogen
- NREL (2018), Fuel Cell and Hydrogen Systems Research
- SCIRO (2018), National Hydrogen Roadmap, Pathways to an economically sustainable hydrogen industry in Australia
- STORE&GO (2018), Report on the costs involved with PtG technologies and their potentials across the EU
- Fraunhofer – ISE (2014), Water Electrolysis: Status and Potential for Development by Tom Smolinka
- Siemens (2019), Efficiency – Electrolysis by Philipp Lettenmeier
- Danish Energy Agency (2021), Technology Data for Renewable Fuels
SCIENTIFIC PAPERS:
- Martín David and al . Advances in alkaline water electrolysers: A review (2019)
- M. Thema and al, Power-to-Gas: Electrolysis and methanation status review (2019)
- Marcela Jimena Lozano Luna, Hydrogen as a Potential Renewable and Secure Source for Energy Supply (2019)
- S. Shiva Kumar and al, Hydrogen production by PEM water electrolysis – A review (2019)
- Hans Böhm and al. Estimating future costs of power-to-gas - a component-based approach for technological learning (2019)
- Joris Proost, State-of-the art CAPEX data for water electrolysers, and their impact on renewable hydrogen price settings (2018)
- Sayed M. Saba and al, The investment costs of electrolysis – A comparison of cost studies from the past 30 years (2017)
- Ligang Wang et al, Power-to-fuels via solid-oxide electrolyzer: Operating window and technoeconomics (2019)
- Domenico Ferrero, Power-to-Gas Hydrogen: techno-economic assessment of processes towards a multi-purpose energy carrier (2016)
- Keith Scott et al, Electrochemical Methods for Hydrogen Production (2019)
- Institute for Sustainable Process Technology (2022), A One-GigaWatt Green-Hydrogen Plant
